No of Pages: 238
Abstract
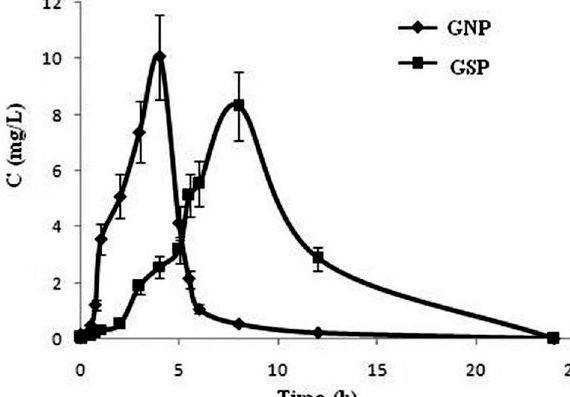
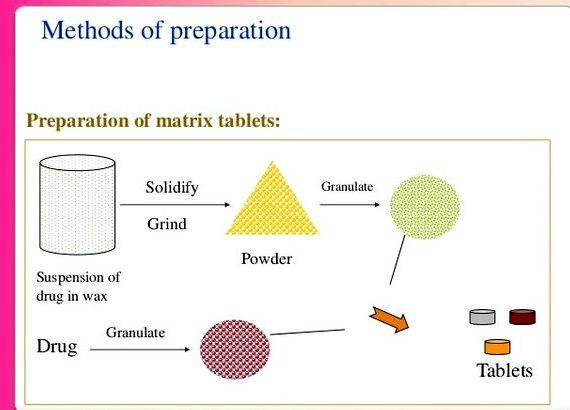
Metronidazole pellets having a power of 88 % were prepared within the rotary fluid-bed spray granulator GPCG-1 using Kollidon 30®. The result of Eudragit L 30 D-55® (enteric-coating) and/or Eudragit NE 30 D® was, individually so that as consecutive layers, evaluated around the discharge of metronidazole as much as thirty percent of putting on weight of dry polymer.These pellets were tested within the USP XXVI apparatus 1 (baskets) at 100 revoltions per minute for just two hrs in acidic medium (.1 N muriatic acidity ) adopted by pH 6.8 phosphate buffer as much as 24 hrs. Greater than ten percent of putting on weight of enteric-coating is needed to effectively block drug release in acidic medium. Pellets coated with Eudragit NE 30 D demonstrated bi-phase dissolution using the greater release rate in acidic medium and sustained release for that remaining 22 hrs in phosphate buffer pH 6.8. The pellets made by the layering of these two film-coatings demonstrated intermediate qualities between delayed release within the acidic stage and sustained release after that. Inside a second stage of the study, the result of three different filler-binders ( microcrystalline cellulose. lactose and dicalcium phosphate) was evaluated around the protection of coated (thirty percent of putting on weight) metronidazole pellets during compression. Enteric-coated pellets manufactured with Eudragit L 30 D-55 created hard tablets however the integrity from the coating was lost as based on the in-vitro release test. Sustained-release pellets manufactured with Eudragit NE 30D, created tablets with lower overall release after 24 hrs compared to uncompressed pellets the decrease was especially apparent for that tablets compressed without filler-binder, however, these tablets didn’t bind.
Another number of pellets was manufactured getting both enteric and sustained release characteristics by coating with Eudragit NE 30D: Eudragit L 30 D-55 (fifteen percent putting on weight for every layer). The tablets from all of these pellets compressed with dicalcium phosphate, along with the uncompressed pellets, satisfy the XXVI USP specifications for delayed release. Hardness of tablets depends, mainly, on the kind of compressed pellets. Individuals prepared from uncoated and enteric-coated pellets produced the toughest tablets. Tablets from dicalcium phosphate demonstrated the cheapest hardness and also the cheapest in-vitro drug release rates therefore, it’s hypothesized the decreased release rate relates to the lengthy disintegration duration of the tablets.




 Methodischer ansatz master thesis proposal
Methodischer ansatz master thesis proposal Artemis sportswear company thesis proposal
Artemis sportswear company thesis proposal Research methodology chapter in phd thesis proposal
Research methodology chapter in phd thesis proposal Duncker hublot kosten dissertation proposal
Duncker hublot kosten dissertation proposal What is local literature and studies in thesis proposal
What is local literature and studies in thesis proposal