Our Guarantees Our Quality Standards Our Fair Use Policy
What Makes UK Essays Different?
- We have a verifiable trading history as a UK registered company (details at the bottom of every page).
- Our Nottingham offices are open to the public where you can meet our team of over 40 full-time staff.
- UK Essays partner with Feefo.com to publish verified customer testimonials – both good and bad!
Ask an Expert FREE
Ask an Expert Index Ask a Question Paid Services
About Our Ask an Expert Service
Our totally free “Ask an Expert” Service allows users to get an answer of up to 300 words to any academic question.
- Questions typically answered within 24 hours.
- All answers are researched and written by fully qualified academics in the question’s subject area.
- Our service is completely confidential, only the answer is published – we never publish your personal details.
- Each professional answer comes with appropriate references.
About Us
More About Us
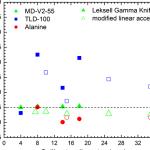
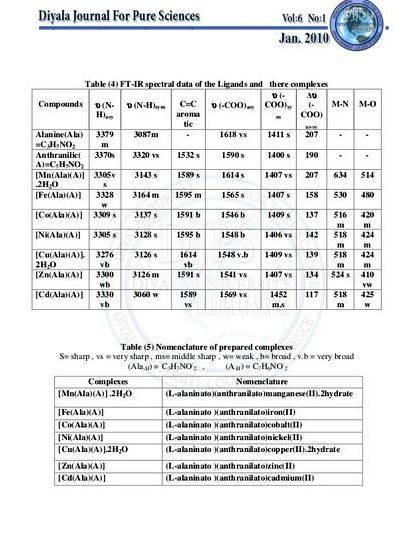
-symmetric NH3angle deformation
-relative intensity. strong
-(Co-N) stretching indicates metal-ligand bond(1)
-(Co-Cl) stretching indicates metal-ligand bond(1)
There are suppose to have a symmetric NH3 stretch, 3169.3 cm-1 and an asymmetric NH3 stretch, 3289.3 cm-1 in the IR spectrum. These two spectrums are important to prove that there are two different chemical conditions for this NH3 ligand in this complex. This condition is actually due to the distortion geometry by chloride ligand.
From 3 of the IR spectrum that we had obtains is that we are able to identify two error in it. First is the peak that going upwards at the region between 2000 cm-1 and 2500 cm-1. This error is due to the FT-IR spectrometry error as it can be shown in the comparison between the second IR spectrums that read by another spectrometry.
Then, the following error is the very strong H2O that is mixed within the compound when we are doing the tablets. This very strong H2O is within the range of 3200 cm-1 to 3800 cm-1 region.
If the intensity of magnetization is negative, the material is said to be diamagnetic. This works when the density of lines that force inside the sample is less than that outside in this material. When it placed in an inhomogeneous magnetic field will tend to move to the region of lowest field. The repulsion that forms from the field will then produce energy in it. So, it is an endothermic process. Magnitude of the attractive force increase with the number of unpaired electrons that contain in the transition metal ion. Thus, the complexes that having a single unpaired d electron will interact less strongly with a magnetic field compared with complexes that have two unpaired electrons. So, complexes that contain no unpaired electrons are said to be diamagnetic and it is only weakly repelled by magnetic field. The figure is also very small as order of -1 to -100×10-6 c.g.s e.m.u. In addition, it does not depend in the field strength and independent on temperature. In this experiment, the chloropentaamminecobalt(III) chloride is a diamagnetic compound.
This Essay is
This essay has been submitted by a student. This is not an example of the work written by our professional essay writers.
Examples of our work
The chloropentaamminecobalt(III) chloride has d6 electron configuration that is high spin. It is zero for the unpaired electrons in the orbital.(100)
If the intensity of magnetization of a paramagnetic is positive, hence δw/δH is negative and such a material will tend to move regions of maximum field strength since this is an exothermic process. The figure for the paramagnetic susceptibility is large and relative large as fall within the range of 100 to 100,000×10-6 c.g.s e.m.u. In addition, it does not depend on magnetic field strength but do depend on temperature. Paramagnetic is a consequence of the interaction of and the spinangular momenta of unpaired electrons with the applied field. Complexes that have no unpaired electron in the orbital will have a magnetic moment that is as strong as it will attract each other stronger in the field. Thus this compound is paramagnetic. In this experiment, the bis(acetylacetonato)oxovanadium(IV) and tris(acetylacetonato)manganese(III) is a paramagnetic compound.
The tris(acetylacetonato)manganese(III) has a d4 low spin of electron configuration with twp unpaired electrons. For the bis(acetylacetonato)oxovanadium(IV) has a d3 electron configuration that has 2 unpaired electrons within the orbital. So, this eventually states that both of the products are paramagnetic. (100)
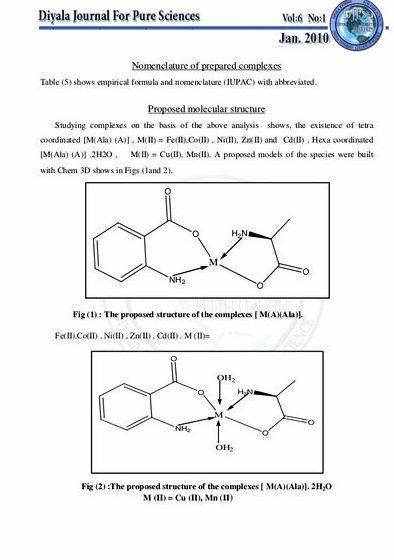
The Shape of the Compounds
The shape for the bis(acetylacetonato)oxovanadium(IV) is actually in a shape of square pyramidal as I had mention in the introduction. The formation of a square pyramidal complex is due to the ligand that influences it. The steric effect between vanadium and the other oxygen bonding will tend to have competed among each other for the spacing with the other ligands in the metal bonding orbital. This effect can be observed in the decrease in the IR stretching frequency of the VO bond when there is a sixth ligand coordinates trans to oxygen. (9)
The shape for penta is in Werner coordination as I have mention also in the introduction. It means that it is in an octahedron shape with a 6 coordination numbers.
The ground state for octahedral complexes Mn(acac)3 which is the product of our experiment 1 of is a 5Eg (t2g 3eg1) position. The black manganese(III) acetylacetonate complex that which is the product of our experiment usually has an octahedral configuration. there actually exists of the Jahn teller distortion. Thus, it will be not a pure octahedral conformation. Then, it will have two forms for this compounds where one is with substantial tetrahedral elongation where two Mn-O bonds at 212 pm, and four at 193 pm and the other with moderate tetragonal compression where the two Mn-O bonds at 195 pm and four at 200 pm.
Namely, The room temperature effective magnetic moments of the manganese(III) complexes with mixed ligands are in the range of 4.76-4.9 μB, which corresponds to four unpaired electrons typical of the d4 system. It is supposed that in mixed-ligand complexes the ligand has localized π-bond and do not favor electron-pairing. The Jahn-Teller effect due to an unequal filling up of t2g and eg orbital yields a distorted octahedral geometry in complex. These complexes have a dark green to green color. The proposed structures of the complexes shown in Fig 3 are consistent with the related data (5).
Request Removal
If you are the original writer of this essay and no longer wish to have the essay published on the UK Essays website then please click on the link below to request removal:
More from UK Essays





 Authoring a phd thesis proposal
Authoring a phd thesis proposal Urban agriculture architecture thesis proposal
Urban agriculture architecture thesis proposal Thesis writing guidelines ppt slides
Thesis writing guidelines ppt slides Phd thesis dissertation database contains
Phd thesis dissertation database contains College of europe thesis proposal
College of europe thesis proposal