Our Guarantees Our Quality Standards Our Fair Use Policy
How Come United kingdom Essays Different?
- There is a verifiable exchanging history as being a United kingdom registered company (details within the finish of every page).
- Our Nottingham offices are suitable for purchase to everybody to satisfy we greater than 40 full-time staff.
- United kingdom Essays partner with Feefo.com to produce verified customer testimonials – both positive and negative!
Ask an expert FREE
Ask an expert Index Ask an issue Compensated Services
About Our Ask an expert Service
Our free of charge “Ask a specialistInch Service enables users to get a solution as much as 300 words for the academic question.
- Questions typically clarified within 24 hrs.
- All solutions are researched and printed by properly accredited academics within the question’s market.
- Our services are totally private, only the solution is printed – we never publish your very own details.
- Each professional answer includes appropriate references.
About Us
More Details On Us
Have the grade
or possibly reimbursement
using our Essay Writing Service!
Essay Writing Service
Eye disease might cause discomfort and anxiety in patients, while using the ultimate anxiety about inadequate vision or even facial problem. Many regions of the eye are relatively inaccessible to systemically administered drug and, consequently topical drug delivery remains preferred route generally. Drug may be sent to treat the precorneal region for such infection as conjunctivitis and blepharitis, so that you can provide intraocular treatment (IOT) using the cornea for illnesses for example glaucoma and uveitis. (Le bourlais C, et al, 1998)
Most ophthalmic drugs are administered topically by means of eyedrops. Although convenient and price-effective, this type of delivery system yields low therapeutic effectiveness because of the dynamics within the lachrimal system (i.e. blinking, lachrymal secretion and nasolachrimal drainage). The low effectiveness necessitates more frequent administration to own preferred therapeutic effect. This may enhance the frequency and harshness of both ocular and systemic undesirable effects. Therefore, you have to develop safer, effective and even more acceptable ocular delivery systems. Delivery systems that could handle releasing the drug within the prolonged manner have interest given that they can raise the ocular residence time. A lift in ocular residence time maximizes the duration for topical or local action additionally to minimizes the systemic undesirable effects. Additionally, a controlled release preparation requires less instillations and thus can result in elevated patient compliance. (Simamora P et al,1998)
Pilocarpine, a parasympathomimitic, remains a miotic loved by open position glaucoma since it improves the out flow of aqueous humour, the drug penetrates the eye well, with miosis beginning 15-30 min after topical application and lasting for 4-8 h (Zimmerman 1981). Pilocarpine ophthalmic drops are administered as a few drops componen dose, with six drops every single day as maximum suggested dosage.
Ocular bioavailability of topically applied pilocarpine is just .1-3% (Lazare and Horlington, 1975) along with the drug needs to be administered as eye-drops 3 or 4 occasions every single day which impairs patient compliance (Kass et al. 1986). The indegent bioavailability pertains to the low lipophilicity of pilocarpineas along with to rapid inadequate the drug inside the precorneal area via drainage and conjunctival absorption. (Sznitowska M et al, 1999)
Chitosan (CS (1,4)-[2-amino -2 deoxy — D- glucan] ) a mixture of glucosamine and N -acetyl-glucosamine, could be a cationic polysaccharide acquired inside the chitin of crustacean shells. Chitosan is biocompatible, biodegradable, not highly toxic and mucoadhesive. (Lin HR et al 2006). The little size and positive charges of chitosan nanoparticles may grow their interaction with negatively billed biological membranes. (En Torre at al 2003 torrado et al, 2004).
During this study we benefit by an method of preparing pilocarpine loaded chitosan nanoparticles. We evaluated the physicochemical portrayal of nanoparticles using particle size, zeta potential, entrapment efficiency in vitro release plus vivo study.
2. Techniques and materials
2.1 Materials
Pilocarpine nitrate was bought in Medicine traders Mumbai India. Chitosan was acquired as gift sample from Indian Ocean Foods, Cochin India. Sodium Tripolyphosphate was bought in Loba Chemicals, Mumbai, Acetic Acidity from Ranbaxy Fine Chemical Limited Mumbai, Acetone, Sodium hydroxide pellets, Potassium dihydrogen orthophosphate were bought in S.D. Fine Chemicals Limited. Mumbai, Ranbaxy Fine Chemical Limited, New Delhi, Himedia Lab, Mumbai correspondingly.
2.2 Method
2.2.1 Preparation of pilocarpine loaded chitosan Nanoparticles
Pilocarpine loaded chitosan Nanoparticles were produced by ionic gelation method. Chitosan solution was prepared in 1%v/v acetic acidity aqueous solution then, TPP in sanitized water at a few mg / ml. Finally, 2 ml of TPP solution was place in 5 ml chitosan solution along with the drug solution were added while using syringe and stirred using mechanical stirrer at 70 levels plus it was further examined as nanoparticles. (Yongmei Xu et al 2003). Facts about formulation proven in table 1 (Jorg Kreuter et al, 1995).
2.3 Check out pilocarpine loaded chitosan Nanoparticles
2.3.1 Percentage yield
Plagiarism-free
Always rapidly
Marked to plain
Percentage practical yield is calculated to discover percentage yield or efficiency connected getting a technique, so it may be helpful for choice of appropriate approach to production. Practical yield was calculated because the weight of nanoparticles retrieved from each batch based on the quantity of the beginning material (Sunit KS et al.2005).
2.3.2 Zeta potential
Zeta potential was measured by using zeta potentiometer (Zeta 3.+ meter, USA). Samples were diluted with KCl (.1mM) and hang into electrophoretic cell in which the electric field of 15.2 V/cm was applied. Each sample was examined in triplicate. (Bekerman, T et al, 2004).
2.3.3 Particle Size Analysis
The size distributions within the volume mean diameters within the suspending particles were measured by dynamic scattering particle size analyzer (Nanotrac Particle Analyzer 150, Microtrac Corporation. PA, USA). All of the different measurement of Nanotrac Particle Analyser 150 is .8 nm
6.54 µm. (Lin Y, et al).
2.3.4 Checking Electron Microscopy (SEM)
Surface morphology within the examples is made a decision employing a checking electron microscope (SEM), Hitachi model SU 1500. The dried samples were put on brass specimen studies, using double sided adhesive tape. Gold-palladium alloy of 1200A knees was coated across the sample using sputter coating unit ( JEOL JFC-Model 1100E, Japan) in Argon at ambient of 8-10 Pascal with plasma current about 20MA. The sputtering ended for nearly a few momemts. The SEM was operated at low speeding up current of roughly 15KV with load current of roughly 80MA. The condenser lens position was maintained between 4.4-5.1 along with the working distance WD=39mm. (Ye, J et al, 2008).
2.3.5 Drug Entrapment Efficiency
The proportion of incorporated pilocarpine nitrate (entrapment efficiency) was resolute spectrophotometrically at 215 nm. After centrifugation within the aqueous suspension, amount of free drug was detected within the supernatant and the quantity of incorporated drug was resolute because of the first drug without any free. (Lin HR et al 2006).
2.3.6 In Vitro Drug Release Studies
The in vitro relieve pilocarpine nitrate inside the formulation was studied through Dialysis membrane-110 (cut-off: 3500 Da) using modified apparatus. The dissolution medium used was freshly prepared simulated tear fluid (pH 7.4). Dialysis membrane-110, formerly drenched overnight within the dissolution medium was associated with one finish in the particularly designed glass cylinder (open each and every side). 5 ml of formulation was place in this setup. The cylinder was placed on a stand and suspended in 50 ml of dissolution medium maintained at 37±€ 1C and so the membrane just touched the receptor medium surface. The dissolution medium was stirred at low speed using magnetic stirrer. Aliquots, of three ml were withdrawn at predetermined occasions and substituted for a similar volume of the medium. The aliquots were suitably diluted while using the receptor medium and examined by Ultra crimson-Vis spectrophotometry at 215 nm. (Srividya B et al, 2001).
2.3.7 In Vivo Studies:
In vivo studies were performed on categories of six male Nz albino rabbits weighing 1.8-2.2 kg, with no symptoms of ocular inflammation or gross abnormalities. The animal’s procedures are fallowed according to CPCSEA guidelines.
Measurement of intra ocular pressure (lOP) reduction
Betamethasone model
The experiments were transported in Nz albino rabbits weighing 1.8-2.2 kg. Ocular hypertension was introduced on by subconjunctival injections of .45 ml betamethasone suspension left eye, repeated weekly a duration of 72 hours. Carrying out a third week of glucocorticoid treatment, a increased ocular pressure, stable for 2 primary days, was acquired. Only rabbits answering the therapy with IOP increases above 25 mmHg were selected for the experiments. Baseline tonometric measurements were taken at various time points a duration of 7.5 h to make sure daily stability within the IOP. Ten µl of Ophtetic ® solution was administered before each measurement to be able to anaesthetize the corneal surface. Transporting out a totally free interval of 24 h, a totally new volume of tonometries was performed to prevent interference from possible corneal damage. The formulations were then tested by instilling 50µl towards the lower conjunctival sac and calculating the IOP after predetermined time occasions. All experiments were transported in the identical hrs during the day to be able to stick to the circadian rhythm (Jorg Kreuter et al, 1995).
Miosis tests
The experiments were transported in male Nz strain albino rabbits weighing 2.2 kg all tests were performed within the same room with constant artificial lighting. After 45 min of acclimatization in restraining boxes.FM-5, 1% Piocarpine Nitrate solution plus a pair ofPercent Piocarpine grew to become tested no under in 6 creatures by instilling some 50µl across the everted lower lid within the left eye. After dosing, the covers were lightly held together for almost any few moments to prevent inadequate dosage form. Measurements of pupil diameter were transported using it . operator, obtaining a micrometer inside a fixed distance inside the rabbits’ eyes. (Jorg Kreuter et al, 1995).
2.3.8 Temporary stability study
This Essay is
This essay remains printed getting students. This isn’t one of the task printed by our professional essay authors.
Types of our work
Details concerning the steadiness of drug substance is an essential part within the systemic method of formulation evaluation. The aim of stability exams will be to provide evidence about how precisely the standard of medicines substance or drug product varies before long under influence of volume of factors for example temperature, humidity and lightweight-weight, also to develop a re-test period for drug substance or even shelves existence for the drug product and suggested storage conditions. Nanoparticle sample was separated into 3 kinds of sets and stored at: 4OC in refrigerator, 37 0C ± 2 OC, /65OC % ± five percent RH in humidity control ovens and 70 levels for 1month.
3. Result and discussion
3.1 Percentage yield
Percent practical yield elevated as the quantity of polymer place in each formulation elevated, although it will not be affected by drug concentration within the formulation. The proportion yield was seen to become 52.5% to 68.%.
3.2 Particle Size
During this study photon correlation spectroscopy was requested routine particle sizing. Table 2 summarizes the outcome acquired for pilocarpine loaded chitosan nanoparticles. The mean diameter of nanoparticle formulations was at the dimensions selection of about252 to557 nm. Checking electron photomicrographs of nanoparticles have been verified in fig. 1a, 1b and 1c. Magnification of seven,500- 20,000 X was applied while taking these photographs. It absolutely was performed to look at the most effective morphology within the particles. Nanoparticles have Smooth textured and scattering nanoparticles the particles were spherical fit.
3.3 Zeta potential
Zeta potential may be the improvement in electrical potential in the tightly bound layer of ions on particle surfaces and bulk liquid where the particles are suspended. It may be quantified by tracking the billed particles once they migrate in current field, as measured in zeta potential analyzer. The zeta potential within the nanoparticles was relating to the +40.6 ± 4.7 to +47.1 ± 1.6mV table 2. The positive surface charges of nanoparticles make sure it is simpler in order to speak with the biological membranes within the eye.
3.4 Drug entrapment efficiency
The quantity of drug bound per 1 ml of nanoparticles was resolute for people formulations along with the values of total entrapment efficiency of drug have been verified in Table 2. It had been observed that because the polymer concentration elevated within the internal phase, a lift in drug entrapment efficiency was seen. The drug encapsulation efficiency elevated from 69.76 % to 83.ten percent.
3.5 In vitro drug release
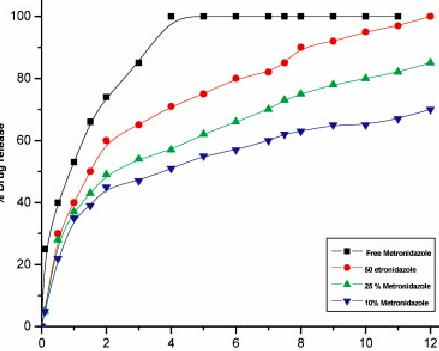
Pilocarpine loaded in eye drops premiered very rapidly, and lots of 95% within the loaded pilocarpine premiered and demonstrated up at in the plateau within 4 h .pilocarpine loaded chitosan nanoparticles proven a preliminary burst release fallowed getting a continuing and sustained releasefor 24 h. fig 2. The in vitro drug relieve FM-1 to FM-6 was seen to become 59.5% to 72.three percent table 3.
3.6 In vivo study:
Intra ocular pressure (IOP)
Since pilocarpine exhibits IOP lowering effects only in hypertensive eyes, hypotensive activity tests needs to be transported on creatures with artificially elevated IOP. During this study, ocular hypertension was caused in rabbits using the approach to (Bonomi et al.1978), comprised of repeated subconjunctival injections of betamethasone. Systems containing nanoparticles, were further tested within the ocularly hypertensive rabbits. IOP values were recorded .5, 1, 2, 4, 6, 8, 10, 12 and round-the-clock after instillation within the selected formulations.
Brought on by IOP decreasing was studied by using tonometer response within the formulations was studied and instead of pure drug and marketed preparation of twoPercent Pilocarpine solutions plus control group no decrease in IOP. IOP activity facts are succumbed table 4. Probably the most period of fact is discovered with FM-5 around 24 hrs. 1% pure drug shows effect around 4hrs plus a pair ofPercent Pilocarpine marketed preparation (eye drops) shows improves the magnitude of response whilst not the time-frame of response figure 3.
In vivo miotic study
Mioitc activity of nanoparticles, 1% pure pilocarpine nitrate plus a pair ofPercent pilocarpine nitrate marketed preparation (eye drop) was studied it shows decrease in pupil diameter brought on by nanoparticles lasts around 24 hrs compared to 1% pure pilocarpine nitrate plus a pair ofPercent marketed preparation of pilocarpine solution table 5, figure 4. The in vivo study of pilocarpine nitrate nanoparticles demonstrates the extended reduction in IOP and miosis effect.
3.7 Stability studies
Stability studies within the prepared nanoparticles were transported out, by storing at 4C in refrigerator, 25±2C/60%±5% RH and 37 C ± 2C, 65% ± 5% RH in humidity control oven for four days. Two parameters namely residual percent drug content plus vitro release studies were transported out. The outcome of drug content after four days have been verified in Table 6. These studies states there’s mortgage loan business drug content after storage for four days at 4 C, 25±2C/60%±5% and 37 C ± 2C/65% ± 5% RH. It had been also states the main one stored at 4 C proven maximum residual drug adopted using the one stored at ambient humidity and temperature and 37 C ± 2C/65% ± 5% RH.
4. Conclusion
The current study can be a acceptable try to formulate nanoparticles of pilocarpine nitrate obtaining a view to improving bioavailability and offering a controlled/ sustained relieve drug and to reduce dosing amounts, frequency of administration, and undesirable effects and the drug efficiency. Nanoparticles were effectively created by ionic gelation method. Chitosan is a good biodegradable polymer that is a good agent for ocular delivery. The effectiveness of TPP increase around 2 Mg/ml improves the entrapment efficiency within the Pilocarpine Nitrate. The in vitro release studies proven biphasic release pattern for people formulations, through getting an initial burst effect, which can be connected using the drug loaded on the top of particles. In vivo study shows probably the most period of response a duration of 24 hrs preserving your IOP at normal average pressure comparatively 1%pure drug plus a pair ofPercent solution of marketed preparation and Stability studies states 4C may be the optimal temperature for storage.
5. Acknowledgement
The authors are grateful to Principal, K.L.E.S’s college of pharmacy Belgaum, Medicine traders for pilocarpine nitrate, Indian ocean foods for polymer, and Shrddha analytical services for offering ale SEM.
Request Removal
If you’re the very first author in the essay with no longer want the essay printed across the United kingdom Essays website then please go here below to request removal:
More from United kingdom Essays




 7 monster theory thesis proposal
7 monster theory thesis proposal Pharmacy inventory system thesis proposal
Pharmacy inventory system thesis proposal Solar inverter design thesis proposal
Solar inverter design thesis proposal Thesis proposal defense presentation philippines embassy
Thesis proposal defense presentation philippines embassy Sample thesis proposal in mathematics
Sample thesis proposal in mathematics