Our Guarantees Our Quality Standards Our Fair Use Policy
How Come United kingdom Essays Different?
- There is a verifiable exchanging history as being a United kingdom registered company (details within the finish of every page).
- Our Nottingham offices are suitable for purchase to everybody to satisfy we greater than 40 full-time staff.
- United kingdom Essays partner with Feefo.com to produce verified customer testimonials – both positive and negative!
Ask an expert FREE
Ask an expert Index Ask an issue Compensated Services
About Our Ask an expert Service
Our free of charge “Ask a specialistInch Service enables users to get a solution as much as 300 words for the academic question.
- Questions typically clarified within 24 hrs.
- All solutions are researched and printed by properly accredited academics within the question’s market.
- Our services are totally private, only the solution is printed – we never publish your very own details.
- Each professional answer includes appropriate references.
About Us
More Details On Us
Printed: 23, March 2015
Metal oxides nanoparticles are usually attractive candidates of functional materials to obtain synthesized inside the nanometer scale. The distinctive characteristics of metal oxides ensure they are probably most likely probably the most varied type of materials, with characteristics covering nearly every part of solid-condition, physics and materials science [1]. Of metal oxides nanoparticles, copper oxide (CuO) has acquired the very best interest due to wide applications, for example solar energy [2], field emission [3], catalysis [4, 5] and gas sensing [6, 7]. Numerous physical and chemical methods are really suggested to synthesize CuO (NPs), for example quick precipitation [8], electrospinning method [9], hydrothermal [10], sol-gel [11], thin-film deposition [12], electrochemical deposition [13] and self-catalytic growth using template [14].
Of individuals methods, quick-precipitation and hydrothermal are the key for safety and eco-friendly [15], and quick-precipitation is especially more inviting because of its cost-effectiveness and straightforward operation. Cupric oxide (CuO) could be a transition metal oxide, a p-type semiconducting material obtaining a narrow band gap of merely one.2 eV [16]. CuO has monoclinic crystalline structure and cell parameters a = .4684 nm, b = .3423 nm, c = .5128 nm and β = 99.54deg [17]. The present study examines well-spread CuO nanosheets, that have been synthesized using the simple quick-precipitation method. This process could be a template free route without requiring warm. Within the preparation process, Copper nitrate three hydrate Cu(NO3)2.3H2O, Polyvinylpyrrolidone (PVP) and sodium hydroxide (NaOH) were selected as copper precursor, stabilizer and accelerator, correspondingly. Sanitized water was applied with the reaction.
Professional
Have the grade
or possibly reimbursement
using our Essay Writing Service!
Essay Writing Service
Within the typical experimental procedure, 2 g of PVP, put in a volumetric flask then added sanitized water to create the amount for the final 100 ml (PVP 2 wt%).
Then 1.45 g Cu(NO3)2.3H2O was dissolved in 60 mL of PVP 2wt%. The answer was added in a round-bottom flask with stirring, the colour of mixture was vibrant blue, then 15 mL NaOH (1M) was quickly put into the mix, nanopowder suspension was created, then stored at 60degC for starters hour. Plenty of black precipitate was created. After being cooled to 70 levels the particles were separated by centrifugation and washed with sanitized water 3 occasions to get rid of the impurities, and dried in oven at 60degC.
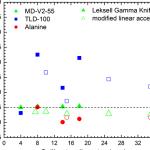
The amount ratio of NaOH comes with a important effect on the very best products. To be able to see the aftereffect in the amount ratio of NaOH, these products were prepared at six volume ratios of NaOH including, 1, 2, 5, 10, 15 and 20 mL NaOH (1M). Inside the finish within the reaction, the pH values of samples were measured (Table. 1). The images of samples after reaction were proven in Fig.1.
Table 1 is unquestionably the variants of pH, maximum absorption wavelengths and colours of products. The pH was elevated from pH 4 to 12.8 with the aid of NaOH (1M). After inclusion of a single, 2, 5 and 10 mL NaOH the colour within the solutions elevated to obtain pale blue and continuing to get unchanged before the finish within the reaction, (Fig. 1) but at greater pH values that have been similar to adding 15 and 20 mL NaOH (1M), the colour of solutions elevated to obtain intense blue then, it altered in situ into black (Fig. 1), that was the indication of CuO nanostructures formation. The pH values elevated moderately adding of merely one, 2, 5 and 10 mL NaOH and elevated significantly after adding 15 mL NaOH. In column absorption wave length (λmax), no peak was observed at low pH values but at greater pH values two peaks were observed at 295 and 356 nm in 15 mL and 280 and 340 nm in 20 mL, correspondingly. The Ultra crimson visible spectra of samples proven in Table 1 have been verified in Fig.2.
5
Fig. 2 is unquestionably the optical absorption characteristics of PVP 2wt % at different volume ratios of NaOH (1M). This figure illustrates that no peak was observed at lower pH values, whereas two peaks at 295 and 356 nm for adding 15 mL and 280 and 340 nm for adding 20 mL, were observed, which proven the development of CuO nanosheets. The observed two peaks in every single curve might the presence of two different shapes or sizes of particles.
6
Fig. 3 shows the XRD pattern within the acquired samples at different volume ratios of NaOH (1, 2, 5 10, 15 and 20 mL). X-ray diffraction (XRD) confirmed the presence of gerhardtite Cu2(OH)3NO3 (Ref Cod# 98-001-7168) at lower pH values (see Fig. 3 (a-d)). This proven that during these volume ratios pure CuO wasn’t created whereas at greater pH values the development of pure CuO was observed (see Figs. 3 (e) and three (f)), all diffraction peaks may be indexed because the monoclinic phase of CuO (Ref Cod# 98-004-8595). The peaks at 2θ values of 32.53, 35.50, 38.67, 46.12, 48.85, 53.43, 58.31, 61.53, 66.33, 67.99, 72.41 and 75.20 match the planes of copper oxide (110), (002), (111), (112), (202), (020), (202), (113), (311), (113), (311) and (004), correspondingly, that are in good agreement with research studies [18]. This result proven the response at lower pH values couldn’t be completed however, the additional of NaOH complete the response.
7
Our Guarantees Our Quality Standards Our Fair Use Policy
How Come United kingdom Essays Different?
- There is a verifiable exchanging history as being a United kingdom registered company (details within the finish of every page).
- Our Nottingham offices are suitable for purchase to everybody to satisfy we greater than 40 full-time staff.
- United kingdom Essays partner with Feefo.com to produce verified customer testimonials – both positive and negative!
Ask an expert FREE
Ask an expert Index Ask an issue Compensated Services
About Our Ask an expert Service
Our free of charge “Ask a specialistInch Service enables users to get a solution as much as 300 words for the academic question.
- Questions typically clarified within 24 hrs.
- All solutions are researched and printed by properly accredited academics within the question’s market.
- Our services are totally private, only the solution is printed – we never publish your very own details.
- Each professional answer includes appropriate references.
About Us
More Details On Us
Printed: 23, March 2015
Metal oxides nanoparticles are usually attractive candidates of functional materials to obtain synthesized inside the nanometer scale. The distinctive characteristics of metal oxides ensure they are probably most likely probably the most varied type of materials, with characteristics covering nearly every part of solid-condition, physics and materials science [1]. Of metal oxides nanoparticles, copper oxide (CuO) has acquired the very best interest due to wide applications, for example solar energy [2], field emission [3], catalysis [4, 5] and gas sensing [6, 7]. Numerous physical and chemical methods are really suggested to synthesize CuO (NPs), for example quick precipitation [8], electrospinning method [9], hydrothermal [10], sol-gel [11], thin-film deposition [12], electrochemical deposition [13] and self-catalytic growth using template [14]. Of individuals methods, quick-precipitation and hydrothermal are the key for safety and eco-friendly [15], and quick-precipitation is especially more inviting because of its cost-effectiveness and straightforward operation. Cupric oxide (CuO) could be a transition metal oxide, a p-type semiconducting material obtaining a narrow band gap of merely one.2 eV [16]. CuO has monoclinic crystalline structure and cell parameters a = .4684 nm, b = .3423 nm, c = .5128 nm and β = 99.54deg [17]. The present study examines well-spread CuO nanosheets, that have been synthesized using the simple quick-precipitation method. This process could be a template free route without requiring warm. Within the preparation process, Copper nitrate three hydrate Cu(NO3)2.3H2O, Polyvinylpyrrolidone (PVP) and sodium hydroxide (NaOH) were selected as copper precursor, stabilizer and accelerator, correspondingly. Sanitized water was applied with the reaction.
Professional
Have the grade
or possibly reimbursement
using our Essay Writing Service!
Essay Writing Service
Within the typical experimental procedure, 2 g of PVP, put in a volumetric flask then added sanitized water to create the amount for the final 100 ml (PVP 2 wt%). Then 1.45 g Cu(NO3)2.3H2O was dissolved in 60 mL of PVP 2wt%. The answer was added in a round-bottom flask with stirring, the colour of mixture was vibrant blue, then 15 mL NaOH (1M) was quickly put into the mix, nanopowder suspension was created, then stored at 60degC for starters hour. Plenty of black precipitate was created. After being cooled to 70 levels the particles were separated by centrifugation and washed with sanitized water 3 occasions to get rid of the impurities, and dried in oven at 60degC.
The amount ratio of NaOH comes with a important effect on the very best products. To be able to see the aftereffect in the amount ratio of NaOH, these products were prepared at six volume ratios of NaOH including, 1, 2, 5, 10, 15 and 20 mL NaOH (1M). Inside the finish within the reaction, the pH values of samples were measured (Table. 1). The images of samples after reaction were proven in Fig.1.
Table 1 is unquestionably the variants of pH, maximum absorption wavelengths and colours of products. The pH was elevated from pH 4 to 12.8 with the aid of NaOH (1M). After inclusion of a single, 2, 5 and 10 mL NaOH the colour within the solutions elevated to obtain pale blue and continuing to get unchanged before the finish within the reaction, (Fig. 1) but at greater pH values that have been similar to adding 15 and 20 mL NaOH (1M), the colour of solutions elevated to obtain intense blue then, it altered in situ into black (Fig. 1), that was the indication of CuO nanostructures formation. The pH values elevated moderately adding of merely one, 2, 5 and 10 mL NaOH and elevated significantly after adding 15 mL NaOH. In column absorption wave length (λmax), no peak was observed at low pH values but at greater pH values two peaks were observed at 295 and 356 nm in 15 mL and 280 and 340 nm in 20 mL, correspondingly. The Ultra crimson visible spectra of samples proven in Table 1 have been verified in Fig.2.
5
Fig. 2 is unquestionably the optical absorption characteristics of PVP 2wt % at different volume ratios of NaOH (1M). This figure illustrates that no peak was observed at lower pH values, whereas two peaks at 295 and 356 nm for adding 15 mL and 280 and 340 nm for adding 20 mL, were observed, which proven the development of CuO nanosheets. The observed two peaks in every single curve might the presence of two different shapes or sizes of particles.
6
Fig. 3 shows the XRD pattern within the acquired samples at different volume ratios of NaOH (1, 2, 5 10, 15 and 20 mL). X-ray diffraction (XRD) confirmed the presence of gerhardtite Cu2(OH)3NO3 (Ref Cod# 98-001-7168) at lower pH values (see Fig. 3 (a-d)). This proven that during these volume ratios pure CuO wasn’t created whereas at greater pH values the development of pure CuO was observed (see Figs. 3 (e) and three (f)), all diffraction peaks may be indexed because the monoclinic phase of CuO (Ref Cod# 98-004-8595). The peaks at 2θ values of 32.53, 35.50, 38.67, 46.12, 48.85, 53.43, 58.31, 61.53, 66.33, 67.99, 72.41 and 75.20 match the planes of copper oxide (110), (002), (111), (112), (202), (020), (202), (113), (311), (113), (311) and (004), correspondingly, that are in good agreement with research studies [18]. This result proven the response at lower pH values couldn’t be completed however, the additional of NaOH complete the response.
7






 Phd thesis sample proposal writing
Phd thesis sample proposal writing Ielts exam tips writing a thesis
Ielts exam tips writing a thesis Thesis writing guide philippines francais
Thesis writing guide philippines francais Thesis proposal on renewable energy
Thesis proposal on renewable energy High harmonic generation thesis proposal
High harmonic generation thesis proposal