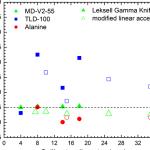
Medical Writing is an essential part of clinical research. Our Medical Authors work carefully with this colleagues within the biostatistical. pharmacovigilance. project management software, and clinical data management teams to provide accurate, timely, and price effective documents towards the greatest ethical and scientific standards.
Our Medical Authors become extra time of the team when you are flexible for your scope, using our expertise to help you using your clinical data and demonstrate the effectiveness of the drug and improve patient safety. There exists a wide breadth of understanding and experience attracted in the pharmaceutical industry, clinical research organizations, and academia. All documents created by Quanticate undergo thorough scientific, record, editorial and qc review.
Quanticate’s medical writing services include clinical and regulatory writing, in addition to scientific communications, education material and medical writing consultancy. These include:
- ICH GCP compliant Clinical Study Reports (CSRs) Phases I to IV, including CSR Synopses for public disclosure
- Study Protocols
- Clinical and non clinical parts of the most popular Technical Document (CTD) including summaries and overviews for EU and US Regulatory Government bodies
- Investigator Brochures
- Patient Safety Narratives
- Patient information including Informed Consent and Patient Brochures
- Pharmacovigilance documents for example Periodic Safety Update Reports
- Standard Operating Procedures (SOPs) covering every aspect of drug development such as the design, conduct and reporting of numerous studies and also the outsourcing of Sponsor responsibilities to some Contract Research Organization (CRO)
- Conference materials (abstracts, poster presentations and slide sets)
- Manuscripts
- Editorial support
- Journal/conference submission
- Product website content (for scientific and patient audiences)
- Educational material for patients, medical professionals and pharmaceutical industry personnel
- Medical marketing reviews and reports
- Literature reviews
- Publication planning





 Ghostwriting services for novels and memoirs
Ghostwriting services for novels and memoirs Dissertations for sale services online
Dissertations for sale services online Member service rep resume writing
Member service rep resume writing Essay writing service recommendation sample
Essay writing service recommendation sample Dissertation service public et union europeenne
Dissertation service public et union europeenne